Drug company Moderna has already begun that process. Once approved the boosters are only.

Booster Shots Fda Advisory Panel Rejects Widespread Pfizer Jabs In Blow To Biden S Plan Abc7 San Francisco
Right now boosters will.
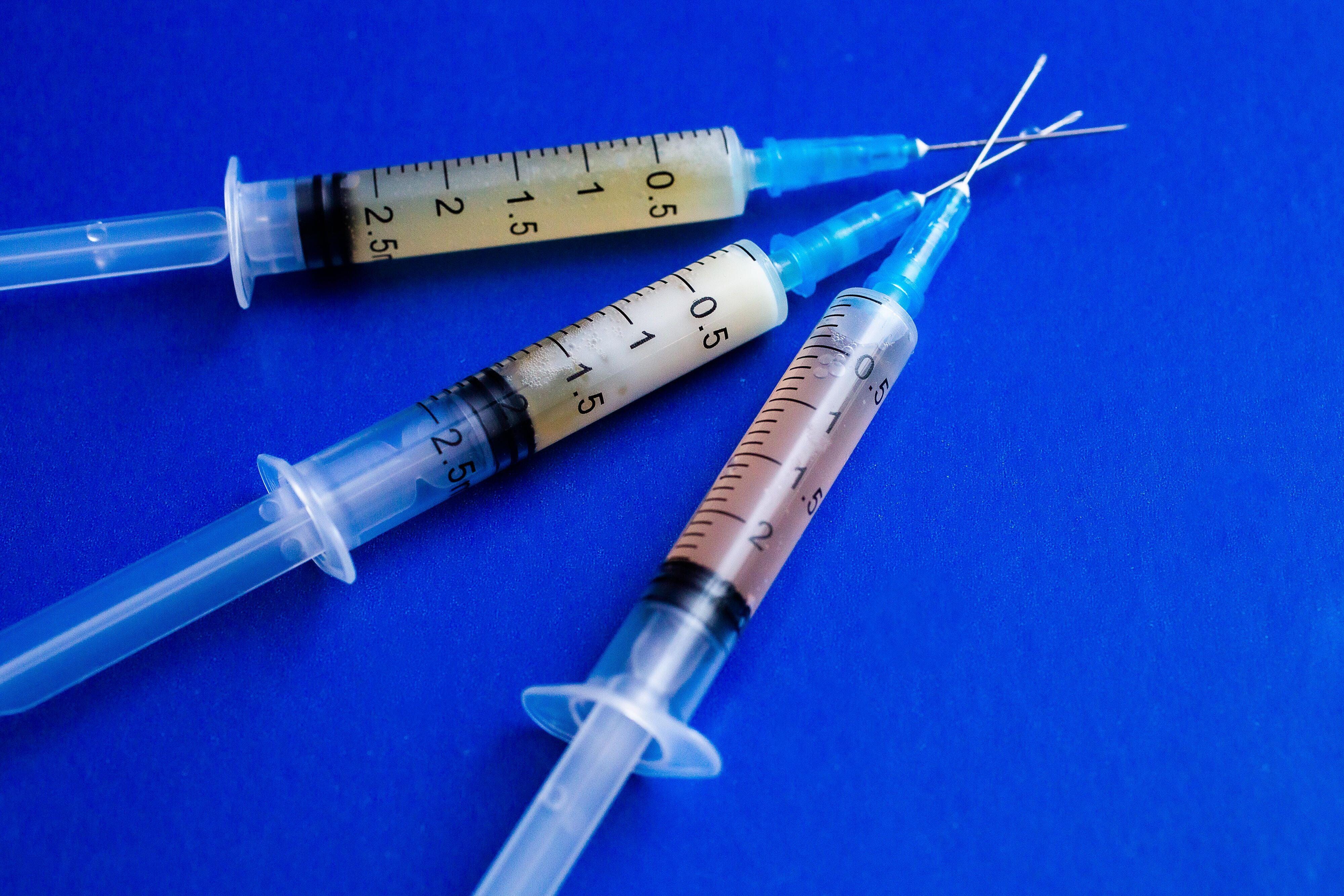
Moderna booster shot time frame. For Pfizer that interval is 21 days. Moderna Booster Shot Time Frame Pfizer Vaccine Protection Wanes CDC. The approval for booster shots is expected in mid-September with vaccines potentially being administered as soon as the week of Monday Sept.
It is likely that healthy people who received two doses of the Moderna or Pfizer vaccine will be eligible for a booster shot but the exact time frame is not yet known. 2 days agoAfter the Pfizer vaccine booster was authorized the CDC and FDA have approved Moderna and JohnsonJohnson booster vaccines prompting a Suffolk community reaction. Moderna booster shot time frame.
Moderna COVID-19 Vaccine and Risk of MyocarditisPericarditis. The time lapse between shot two and three for the mRNA vaccines which the FDA says should be a minimum of six months can also explain the milder side effects that some have. The eight-month time frame is most likely based on findings from both the US.
But Modernas booster shots will target this and other emerging strains. The company said it would work with public health officials on a plan for a booster shot for eight months or longer after the first dose of its vaccine but there is no time frame in place. The longest duration the Pfizer and Moderna vaccines have been studied for.
What to know about coronavirus. Doctors share everything you need to know about the timing of the COVID booster or regular COVID vaccine and flu shot. Can you get our COVID booster and fu shot at the same time.
According to Bancel the company is currently working on two variant-specific booster vaccines. Moderna released new data on its vaccines efficacy on Wednesday showcasing a lower risk of breakthrough infections among recently vaccinated individuals making a case for the need for. And abroad looking at how the vaccines have held up over time and whether they can stand up to the.
The Pfizer vaccine is the first to receive FDA approval while the Moderna and Johnson Johnson vaccines are available under an emergency use authorization. Booster shots for recipients of the Johnson Johnson vaccines also will likely be needed according to HHS although the time frame will differ because administration of that vaccine. Ad injection has an FDAapproved autoinjector.
This year that time frame could overlap with the period when many Americans may become eligible for a booster dose of the coronavirus vaccine. For Moderna 28 days. Per the new plan agencies are preparing to offer booster shots in September to all eligible Americans starting eight months after their second dose of Pfizers or Modernas vaccine.
In fact Moderna recently announced it is working to develop a joint flu shot and COVID-19 booster combining its existing COVID vaccine with an experimental flu vaccine. Study Shows This means at this time only those who received the pfizer vaccine can get the pfizer booster. Data Supporting Need for a Booster Shot.
For children ages 12 to 15. Those time frames come from how the vaccines were administered in clinical trials meaning the only data available look at how well. Studies show that after getting vaccinated against COVID-19 protection against the virus may decrease over time and be less able to protect.
Ad injection has an FDAapproved autoinjector. The US Food and Drug Administration is having a busy month authorizing a booster shot of Modernas COVID-19 vaccine on Wednesday for adults age 65 and older those who are at risk of. News of the potential combo shot comes as the Food and Drug Administration mulls approval for Modernas COVID-19 booster vaccine.
Regulators approved a third dose of. PHOENIX Booster shots of Moderna and Johnson Johnsons COVID-19 vaccine were unanimously recommended Friday by the FDAs Vaccine and Related Biological Products Advisory. Time and again experts have said pharmaceutical companies will be able to adjust their vaccines to deal with new strains of the virus.
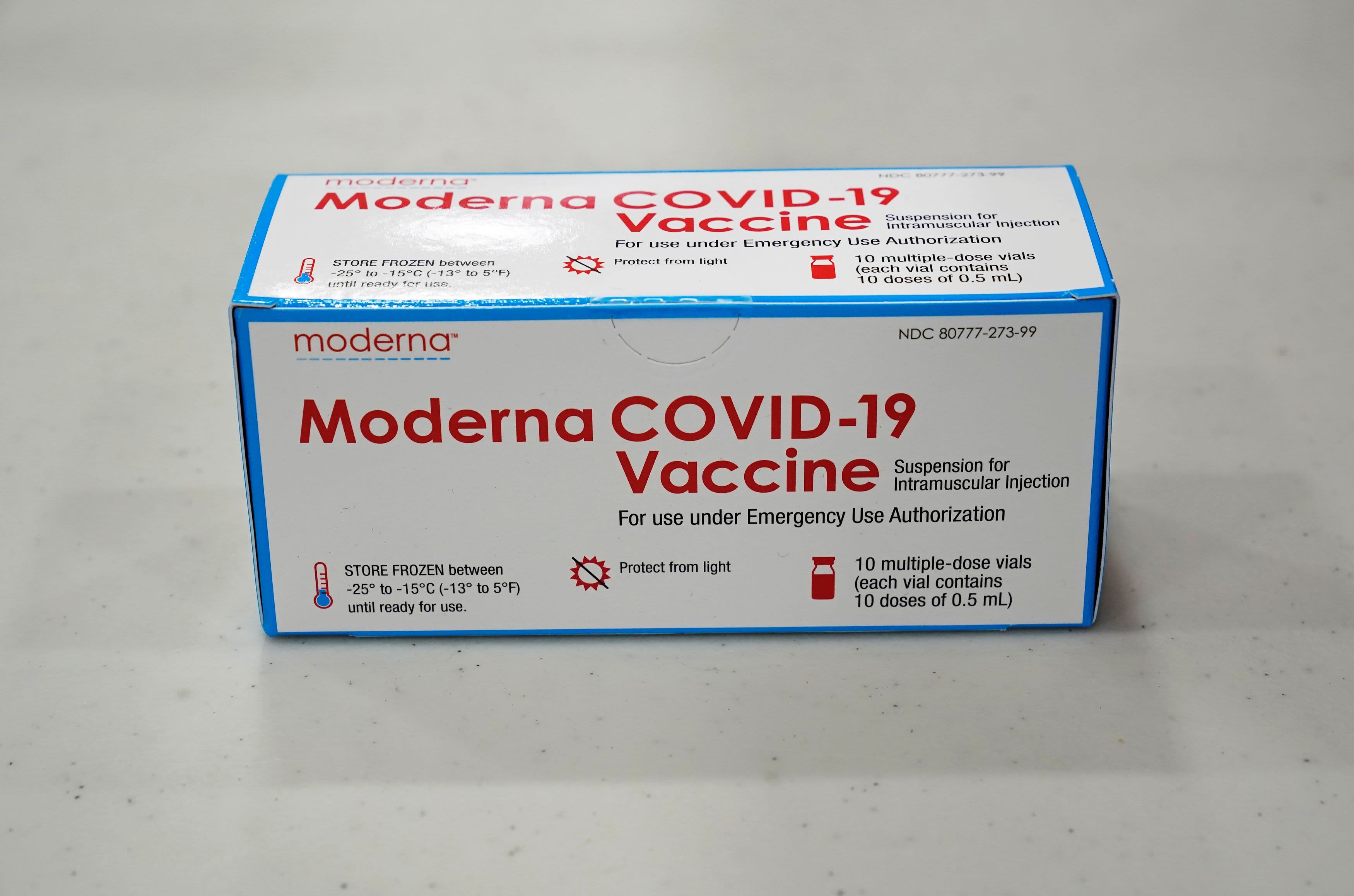
Moderna Looks To Test Covid 19 Booster Shots A Year After Initial Vaccination

Moderna Plans To Have Covid Vaccine Booster Shot Ready By Fall Cbs News

Frequently Asked Questions And Answers About Moderna Covid 19 Vaccine Bangkok Hospital

Moderna Covid Vaccine Booster Shot The Latest On Approval Process Cnet

Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr

Moderna Seeks Fda Approval For Booster Shots Of Its Covid 19 Vaccine Healthcare Finance News

Covid Booster Shots Everything You Need To Know The Brink Boston University
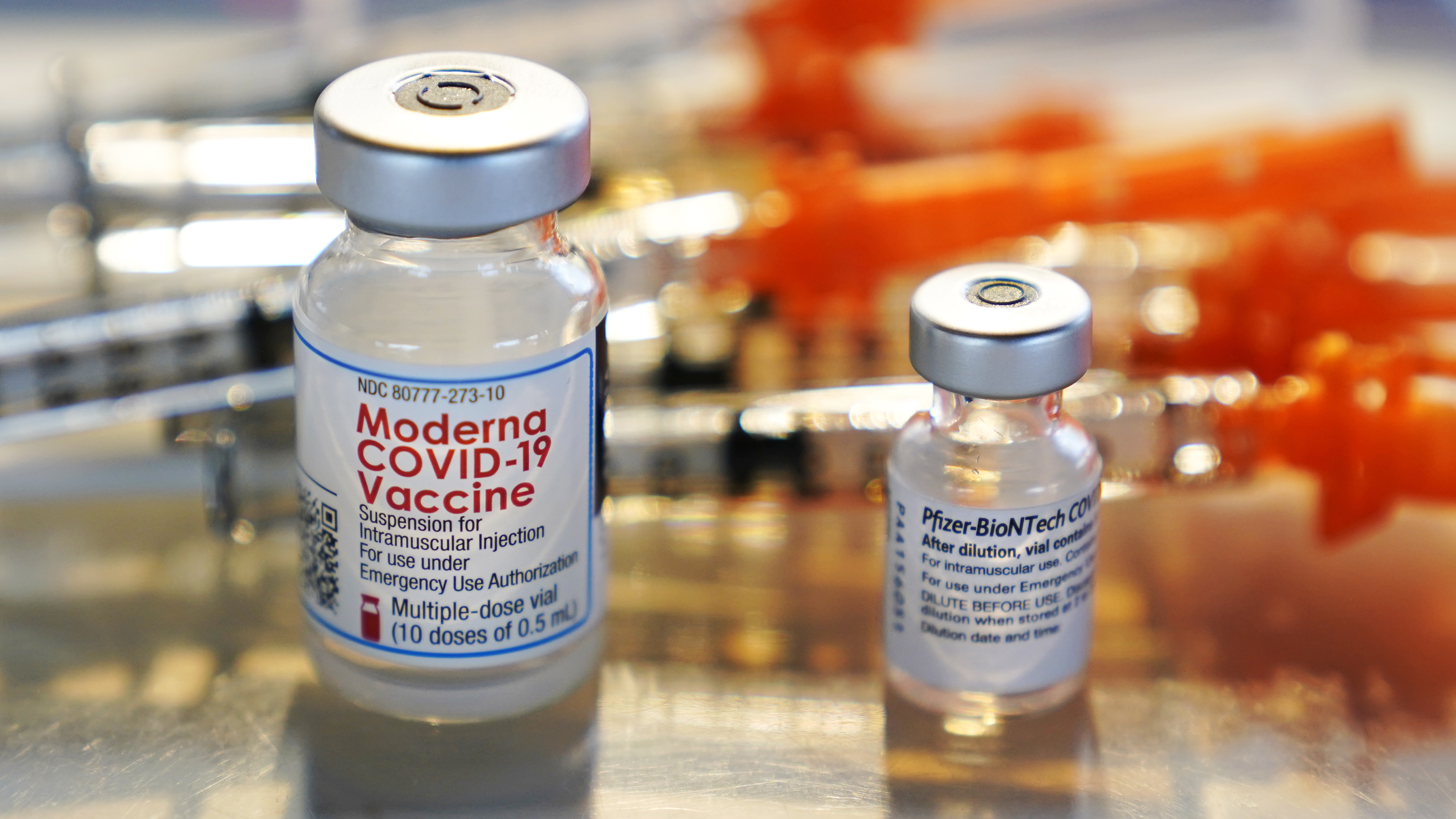
The Biden Administration May Recommend Covid Boosters After 8 Months Npr

Moderna Covid Vaccine Booster Produces Robust Response Against Delta
/cloudfront-us-east-1.images.arcpublishing.com/gray/NZPGPXPQUJDSVC7FJIONPNSKWU.jpg)
Virginia Teen Claims She Received 6 Doses Of Pfizer Vaccine
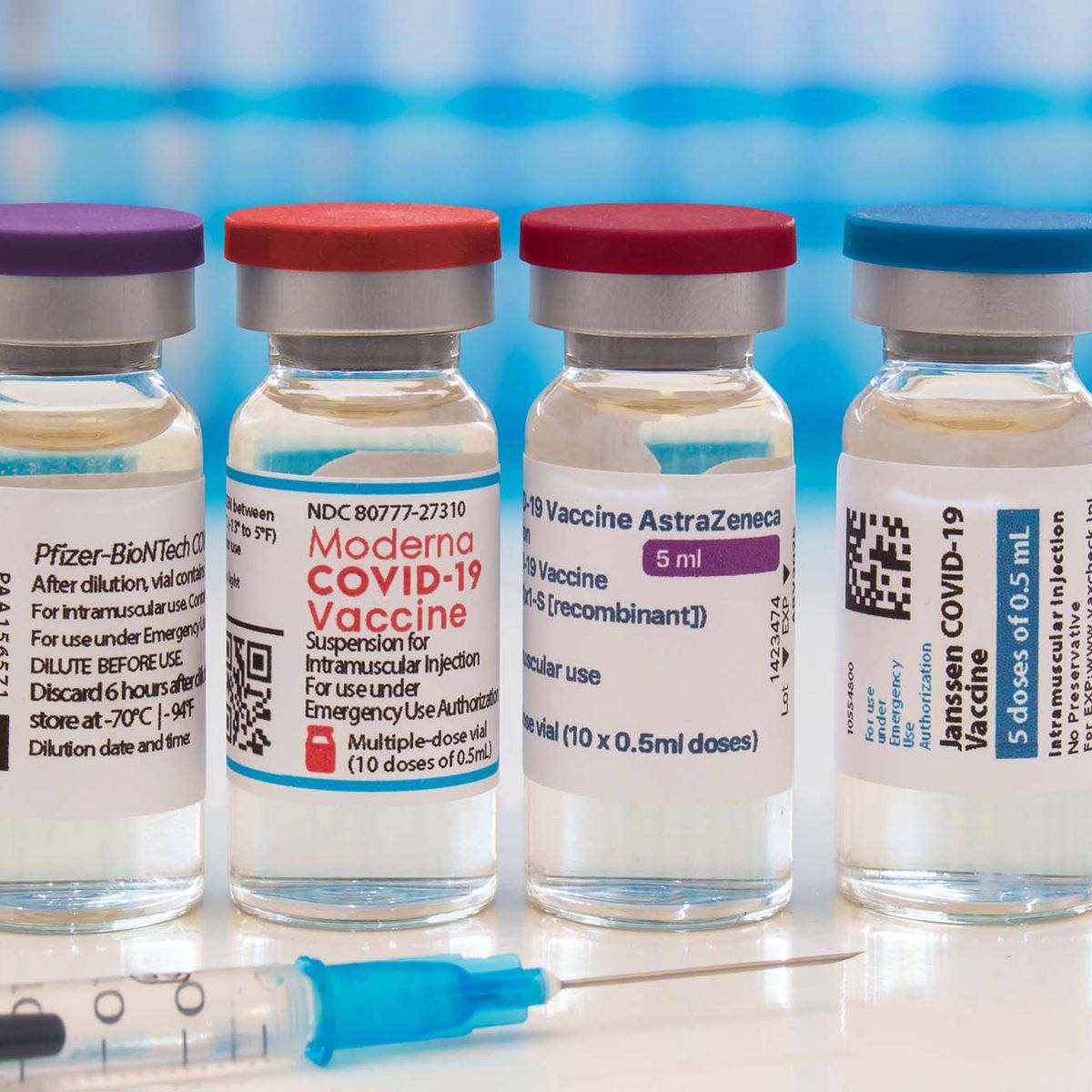
Covid Booster Shots Everything You Need To Know The Brink Boston University
With Pfizer Vaccine Boosters Now Available What S Next For Moderna And J J What To Know Cnet
Covid 19 Booster Shots Health Mil

Covid 19 Vaccine Update Lasalle County

Boosters For Moderna And J J Recipients Not Up For Debate At C D C Panel The New York Times

Covid Booster Shot Moderna Says Vaccine Generates Promising Immune Response Against Variants

People Are Getting Moderna Boosters Anyway Medpage Today
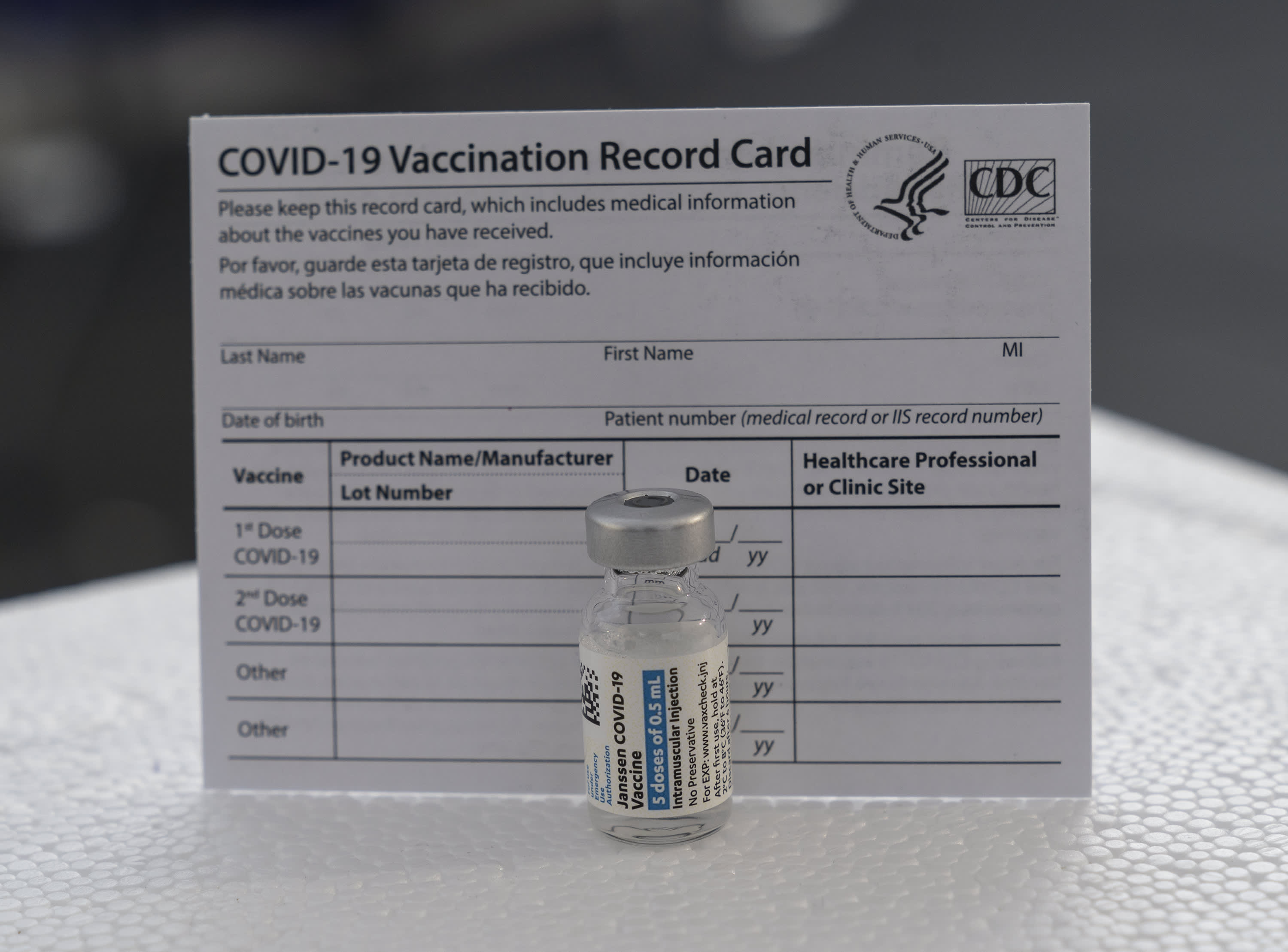
What People Who Got J J Covid Vaccine Need To Know About Boosters
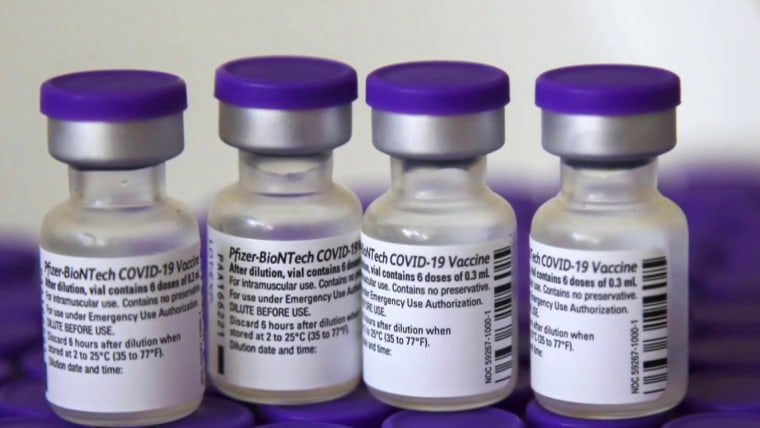
Fda Advisory Group Recommends Moderna Booster For Emergency Authorization

ConversionConversion EmoticonEmoticon